 |
| May 23, 2023 | Volume 19 Issue 20 |
Designfax weekly eMagazine
Archives
Partners
Manufacturing Center
Product Spotlight
Modern Applications News
Metalworking Ideas For
Today's Job Shops
Tooling and Production
Strategies for large
metalworking plants
Liquid car fuels derived from solar power in a single step with artificial leaves
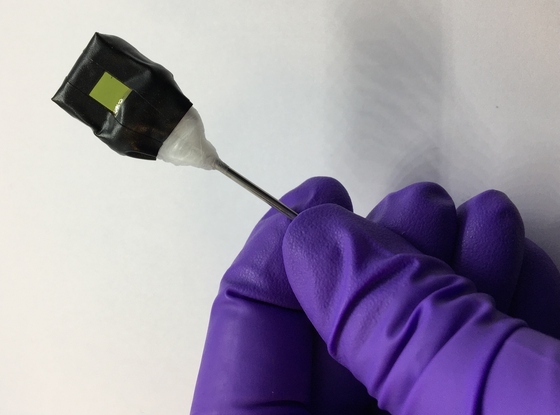
A standalone artificial leaf attached to a metal rod support. The photoanode side (green square) is visible in the photograph. [Credit: Motiar Rahaman/University of Cambridge]
Researchers in the UK have developed a solar-powered technology that converts carbon dioxide and water into liquid fuels that can be added directly to a car's engine as drop-in fuel.
The researchers, from the University of Cambridge, harnessed the power of photosynthesis to convert CO2, water, and sunlight into multicarbon fuels -- ethanol and propanol -- in a single step. These fuels have a high energy density and can be easily stored or transported.
Unlike fossil fuels, these solar fuels produce net zero carbon emissions and are completely renewable, and unlike most bioethanol, they do not divert any agricultural land away from food production.
While the technology is still at laboratory scale, the researchers say their "artificial leaves" could be an important step in the transition away from a fossil fuel-based economy. The results are reported in the journal Nature Energy.
Bioethanol is touted as a cleaner alternative to gasoline, because it is made from plants instead of fossil fuels. Most cars and trucks on the road today run on gasoline containing up to 10% ethanol (E10 fuel). The United States is the world's largest bioethanol producer: according to the U.S. Department of Agriculture, almost 45% of all corn grown in the United States is used for ethanol production.
"Biofuels like ethanol are a controversial technology, not least because they take up agricultural land that could be used to grow food instead," said Professor Erwin Reisner, who led the research.
For several years, Reisner's research group, based in the Yusuf Hamied Department of Chemistry, has been developing sustainable, zero-carbon fuels inspired by photosynthesis -- the process by which plants convert sunlight into food -- using artificial leaves.
To date, these artificial leaves have only been able to make simple chemicals, such as syngas, a mixture of hydrogen and carbon monoxide that is used to produce fuels, pharmaceuticals, plastics, and fertilizers. However, to make the technology more practical, it would need to be able to produce more complex chemicals directly in a single solar-powered step.
To make syngas, two light absorbers on the artificial leaf, similar to the molecules in plants that harvest sunlight, are combined with a catalyst made from the naturally abundant element cobalt. When the device is immersed in water, one light absorber uses the catalyst to produce oxygen. The other carries out the chemical reaction that reduces carbon dioxide and water into carbon monoxide and hydrogen, forming the syngas mixture. In previous research, the researchers also discovered that their light absorbers work even under the low levels of sunlight on a rainy or overcast day.
In the newly developed technology, the artificial leaf can directly produce clean ethanol and propanol without the need for the intermediary step of producing syngas.
To do this, the researchers developed a copper and palladium-based catalyst. The catalyst was optimized in a way that allowed the artificial leaf to produce more complex chemicals, specifically the multicarbon alcohols ethanol and n-propanol. Both alcohols are high-energy-density fuels that can be easily transported and stored.

A photoreactor with an artificial leaf working under solar irradiation. [Credit: Motiar Rahaman/University of Cambridge]
Other scientists have been able to produce similar chemicals using electrical power, but this is the first time such complex chemicals have been produced with an artificial leaf using only the energy from the Sun.
"Shining sunlight on the artificial leaves and getting liquid fuel from carbon dioxide and water is an amazing bit of chemistry," said Dr. Motiar Rahaman, the paper's first author. "Normally, when you try to convert CO2 into another chemical product using an artificial leaf device, you almost always get carbon monoxide or syngas, but here, we've been able to produce a practical liquid fuel just using the power of the Sun. It's an exciting advance that opens up whole new avenues in our work."
At present, the device is a proof of concept and shows only modest efficiency. The researchers are working to optimize both the light absorbers (so they can better absorb sunlight) and the catalyst (so it can convert more sunlight into fuel). Further work will also be required to make the device scalable, so it can produce large volumes of fuel.
"Even though there's still work to be done, we've shown what these artificial leaves are capable of doing," said Reisner. "It's important to show that we can go beyond the simplest molecules and make things that are directly useful as we transition away from fossil fuels."
Source: University of Cambridge
Published May 2023
Rate this article
View our terms of use and privacy policy
